Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes

Molecules | Free Full-Text | Ni Oxidation State and Ligand Saturation Impact on the Capability of Octaazamacrocyclic Complexes to Bind and Reduce CO2
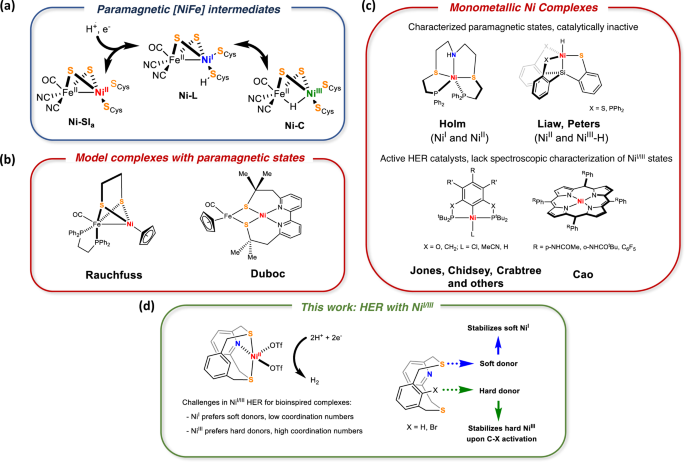
Characterization of paramagnetic states in an organometallic nickel hydrogen evolution electrocatalyst | Nature Communications
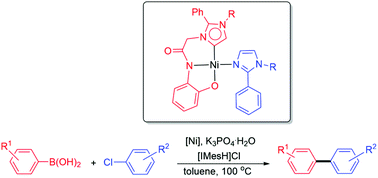
Nickel(ii) complexes containing tridentate ONCi ligands (i = abnormal N-heterocyclic carbene donors) and their catalytic application in Suzuki–Miyaura coupling reaction - Dalton Transactions (RSC Publishing)
![Which of the coordination complexes listed below is the most stable (i.e. has the highest formation constant)? a. [NiEDTA]2- b. [Ni(CO)6]2+ c. [Ni(OH2)6]2+ d. [Ni(NH3)6]2+ e. NiCl2 | Homework.Study.com Which of the coordination complexes listed below is the most stable (i.e. has the highest formation constant)? a. [NiEDTA]2- b. [Ni(CO)6]2+ c. [Ni(OH2)6]2+ d. [Ni(NH3)6]2+ e. NiCl2 | Homework.Study.com](https://homework.study.com/cimages/multimages/16/1.1.17267916333899178067.png)
Which of the coordination complexes listed below is the most stable (i.e. has the highest formation constant)? a. [NiEDTA]2- b. [Ni(CO)6]2+ c. [Ni(OH2)6]2+ d. [Ni(NH3)6]2+ e. NiCl2 | Homework.Study.com

Nickel(II) N‐Heterocyclic Carbene Complexes: Versatile Catalysts for C–C, C–S and C–N Coupling Reactions - Junquera - 2017 - European Journal of Inorganic Chemistry - Wiley Online Library

Ni(I)–Alkyl Complexes Bearing Phenanthroline Ligands: Experimental Evidence for CO2 Insertion at Ni(I) Centers | Journal of the American Chemical Society
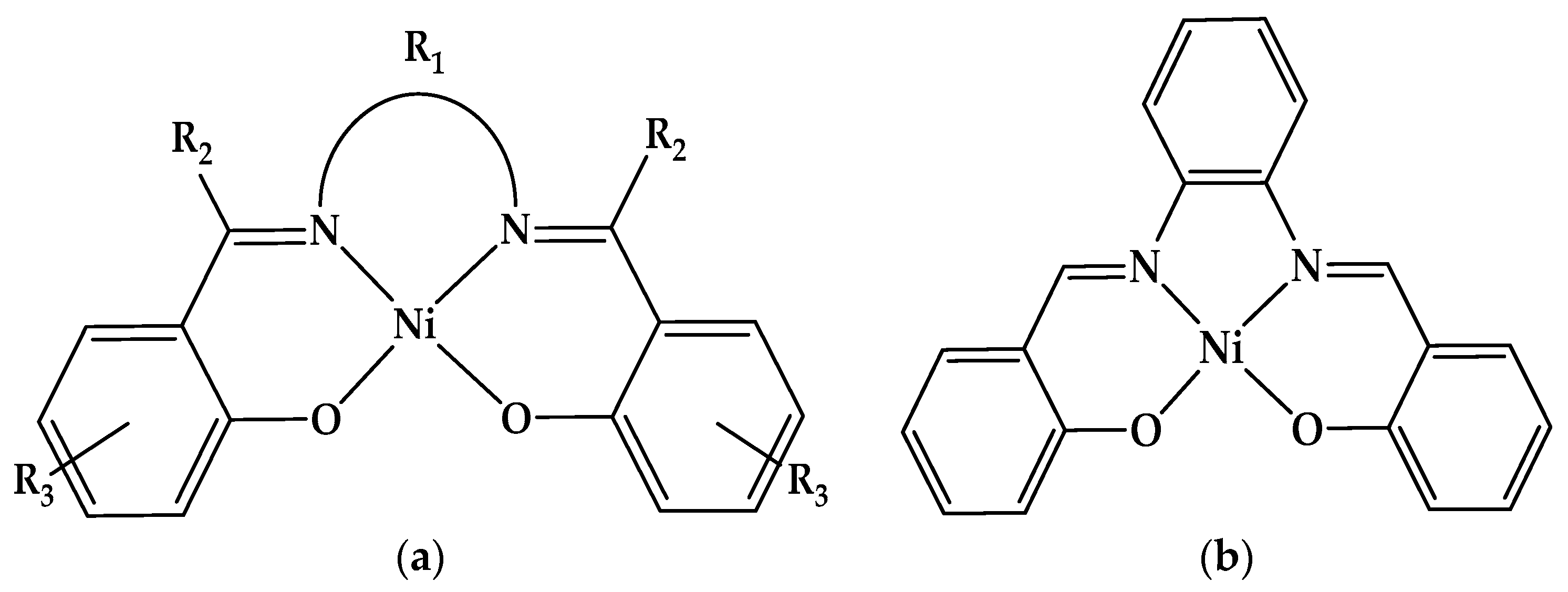
Molecules | Free Full-Text | Ni(II)-Salophen—Comprehensive Analysis on Electrochemical and Spectral Characterization and Biological Studies

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes

Photoactive Nickel Complexes in Cross‐Coupling Catalysis - Wenger - 2021 - Chemistry – A European Journal - Wiley Online Library

Synthesis, characterization and application of nickel(II) complexes modified with N,N′,N″-pincer ligands - ScienceDirect

Nickel Complexes with New Bidentate P,N Phosphinitooxazoline and -Pyridine Ligands: Application for the Catalytic Oligomerization of Ethylene | Inorganic Chemistry
Complexes of Ni(i): a “rare” oxidation state of growing importance - Chemical Society Reviews (RSC Publishing)

A Series of 4- and 5-Coordinate Ni(II) Complexes: Synthesis, Characterization, Spectroscopic, and DFT Studies | Inorganic Chemistry

E735: Complex Ions and Precipitates – Nickel(II) compounds | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder