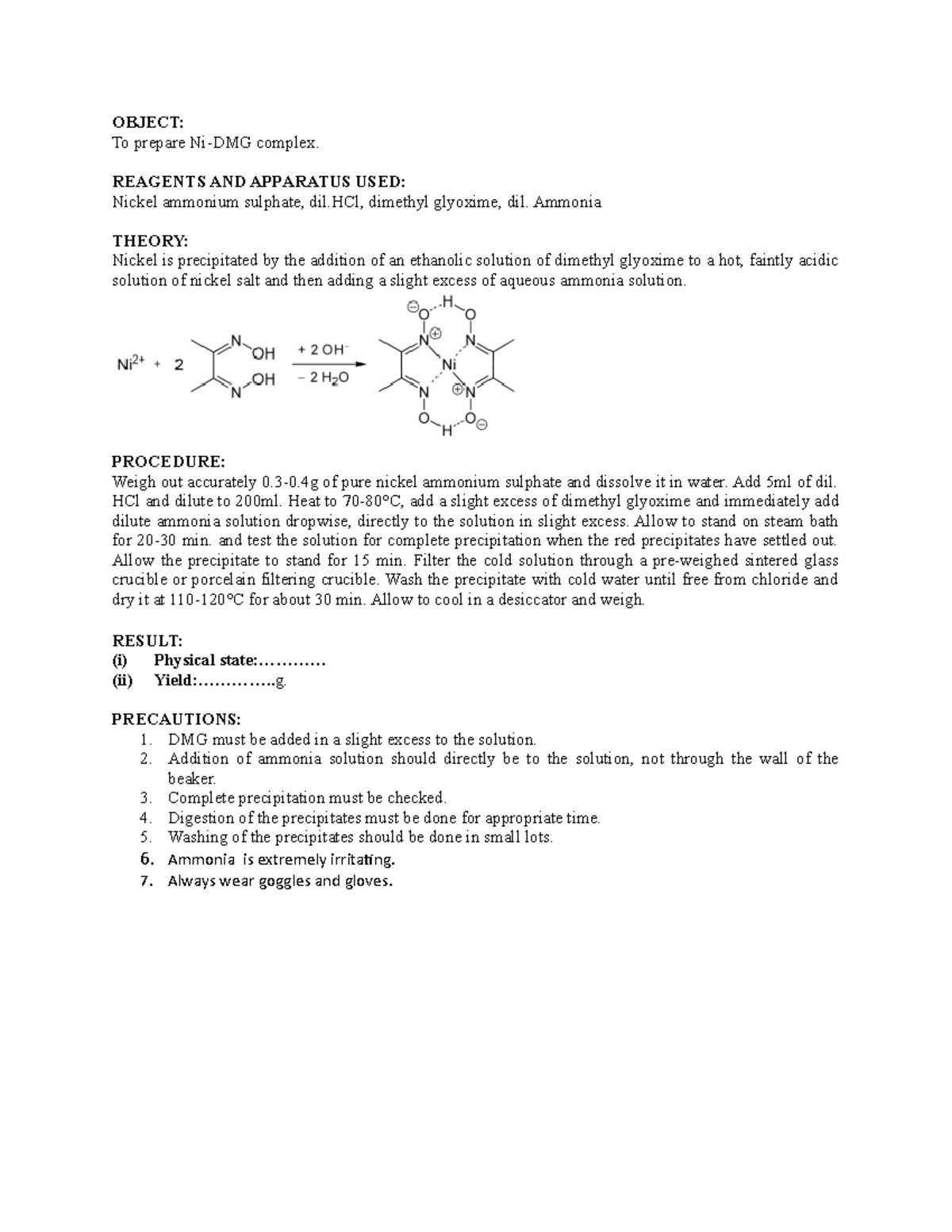
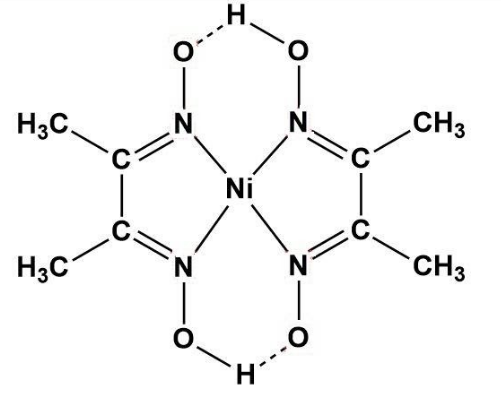
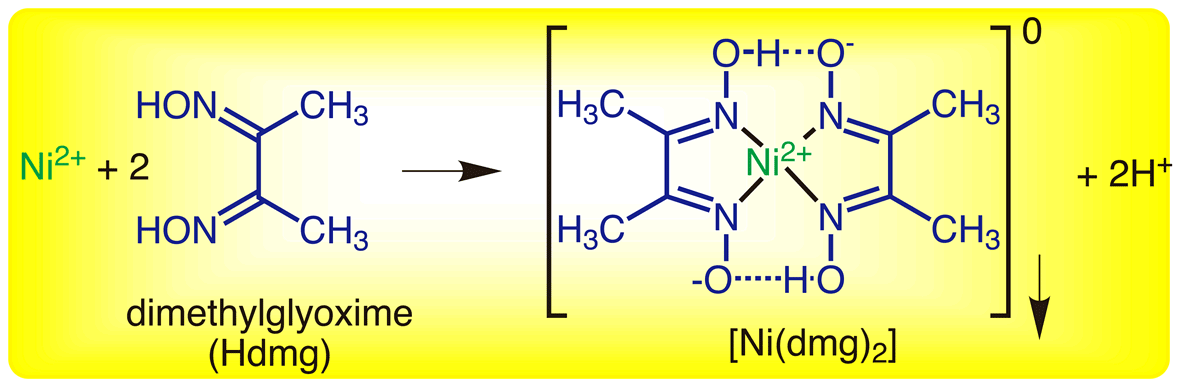
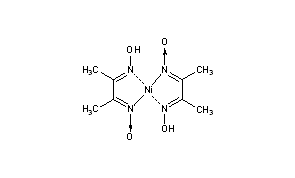
Ni-DMG complex - B Sc Inorganic Practicals - OBJECT: To prepare Ni-DMG complex. REAGENTS AND - Studocu

Structural Properties of Nickel Dimethylglyoxime at High Pressure: Single-Crystal X-ray Diffraction and DFT Studies | The Journal of Physical Chemistry C

a) What is the name and molecular formula of the reagent responsible for the formation of the nickel complex ions? | Homework.Study.com

Ultratrace Detection of Nickel(II) Ions in Water Samples Using Dimethylglyoxime-Doped GQDs as the Induced Metal Complex Nanoparticles by a Resonance Light Scattering Sensor | ACS Omega

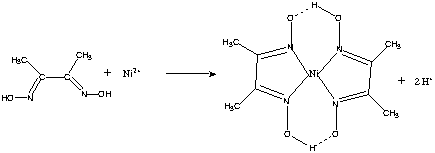


![The IUPAC name of the complex Ni[C(4)H(7)O(2)N(2)] formed by the react The IUPAC name of the complex Ni[C(4)H(7)O(2)N(2)] formed by the react](https://d10lpgp6xz60nq.cloudfront.net/physics_images/RS_P2_CHM_C09_E01_015_S01.png)
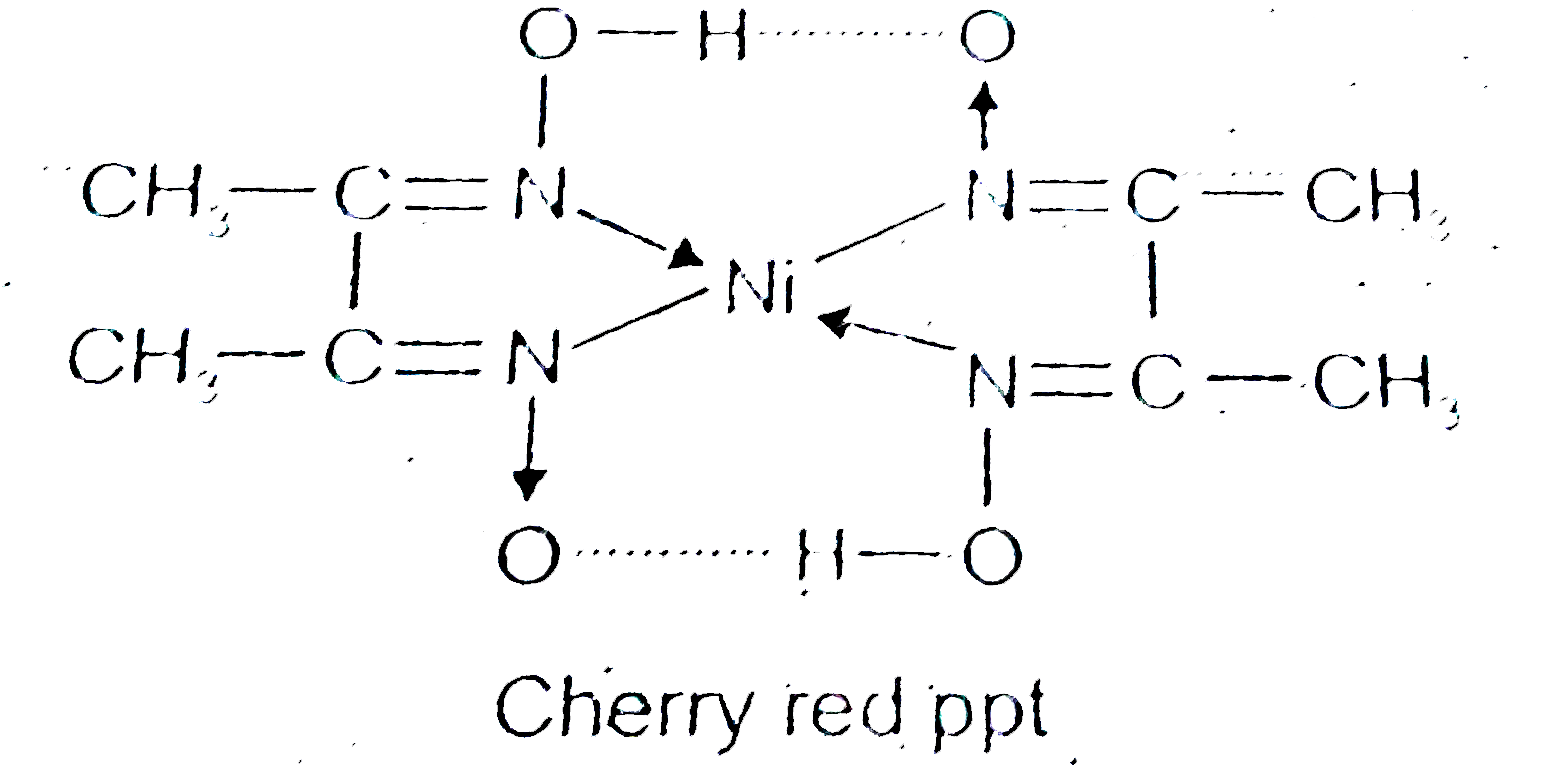






![What is the coordination number of Ni in the nickel-DMG complex?A.\\[2\\] B.$3$ C.$6$ D.$4$ What is the coordination number of Ni in the nickel-DMG complex?A.\\[2\\] B.$3$ C.$6$ D.$4$](https://www.vedantu.com/question-sets/ceab1c4b-aba0-43e1-b397-f3dbf0d0d9bd5238124797968177434.png)



