Effect of time on Ni and Cd leaching recovery (temperature=45 °C, NH4OH... | Download Scientific Diagram
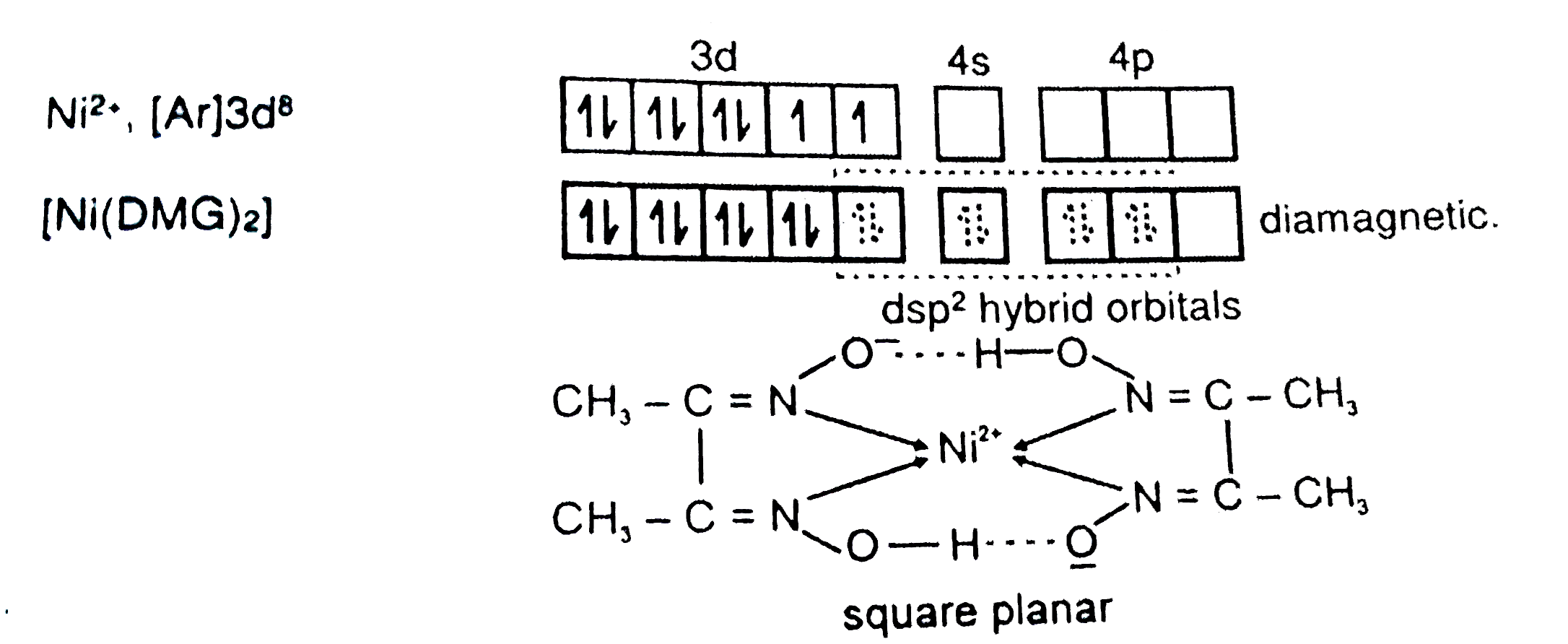
27. DMG +NiCl2+NH4OH makes Complex a+ NH4Cl+H2O. What is complex a and find the hybridisation magnetic character and Oxidation state of Nickel in complex a ?

It is an experimental fact that:- DMG+Ni(II) salt+NH_4OH→ Red precipitate Which of the following ... - YouTube

Effect of temperature on Ni and Cd leaching recovery (NH4OH / (NH4)2CO3... | Download Scientific Diagram

E735: Complex Ions and Precipitates – Nickel(II) compounds | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

Shape-controlled synthesis of Ni(OH)2/NiO nanowalls by surface reaction of Ni foil in aqueous NH4OH - ScienceDirect
What is meant by aqueous ammonia solution? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

Alkaline leaching of nickel bearing ammonium jarosite precipitate using KOH, NaOH and NH4OH in the presence of EDTA and Na2S | Semantic Scholar

Scanning Electron Microscope image of Ni-BTC MOFs. (a) Ni-BTC Anl ; (b)... | Download Scientific Diagram
29. NiCl2 + NH4OH + dimethylglyoxime ——> A ( complex ) Incorrect statement for complex A is /are 1 Coordination number of metal ion is 4 2 Two five membered and two
What is nh3 aqueous solution?. NH3 aqueous means aqueous solution of… | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

Epitaxial N–H-doped Ni1−x O and Ni2O3 with special planar defects by pulsed laser ablation of metallic Ni in aqueous ammonia | Applied Physics A

Separation of Ni, Co, and Mn from Spent LiNi0.5Mn0.3Co0.2O2 Cathode Materials by Ammonia Dissolution | ACS Sustainable Chemistry & Engineering



/fusion-NH4OH-generation-system-process-flow-diagram.png?width=710&name=fusion-NH4OH-generation-system-process-flow-diagram.png)