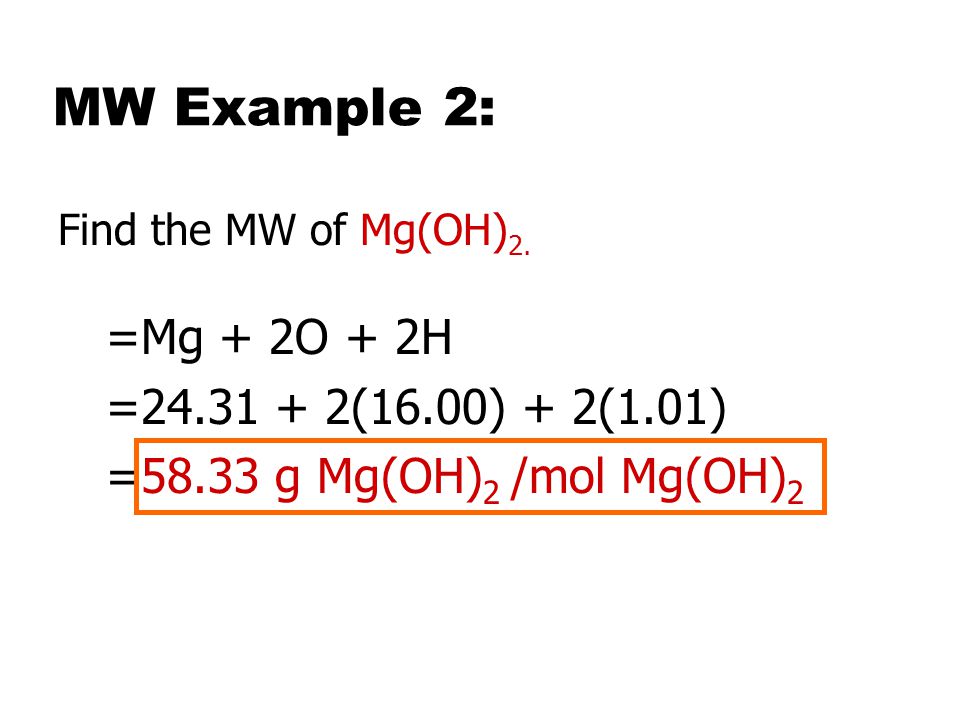
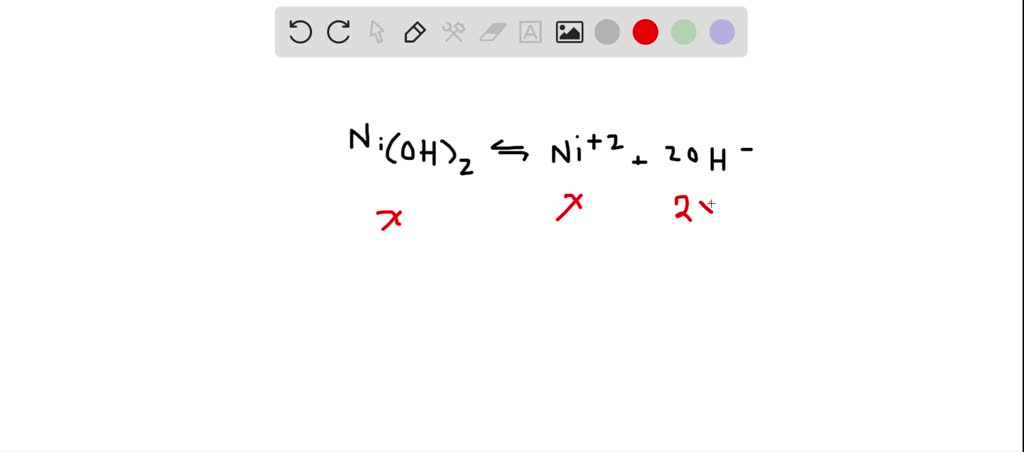
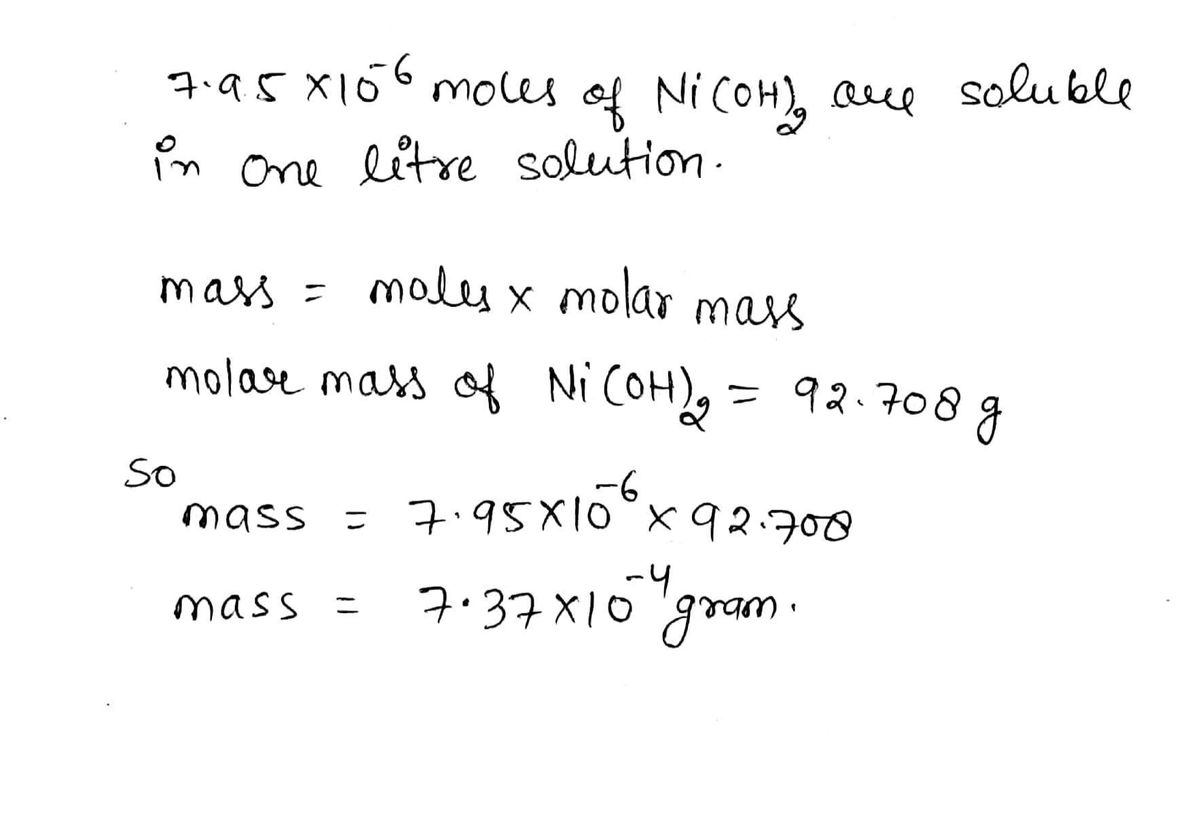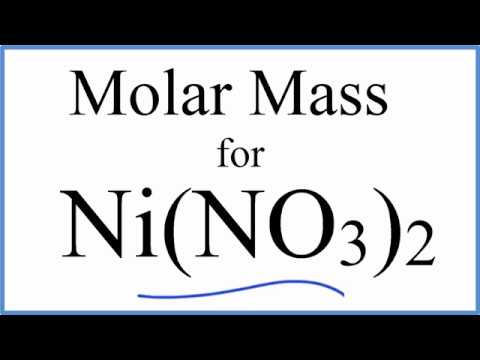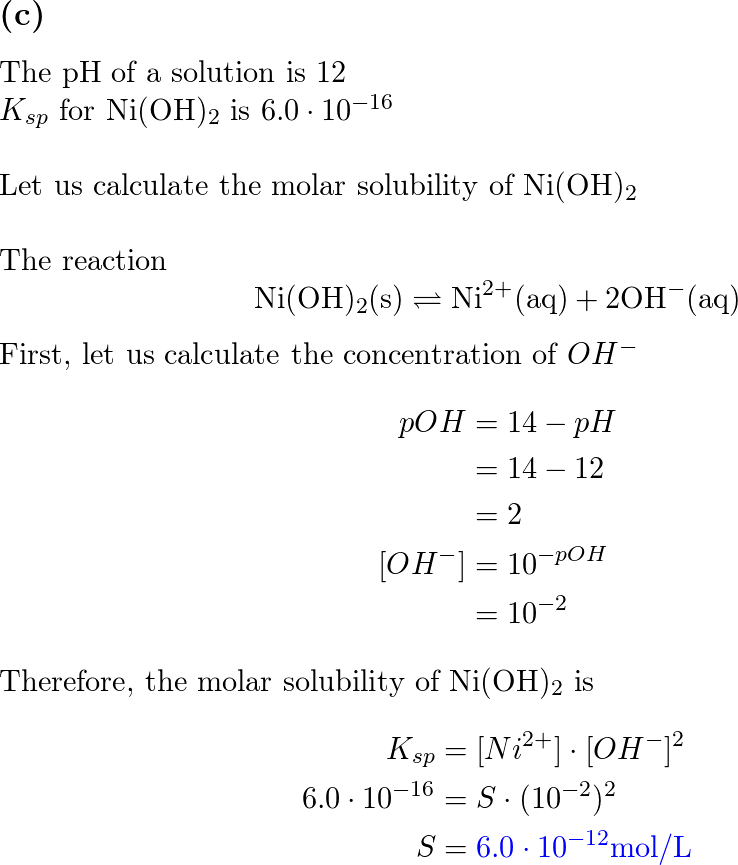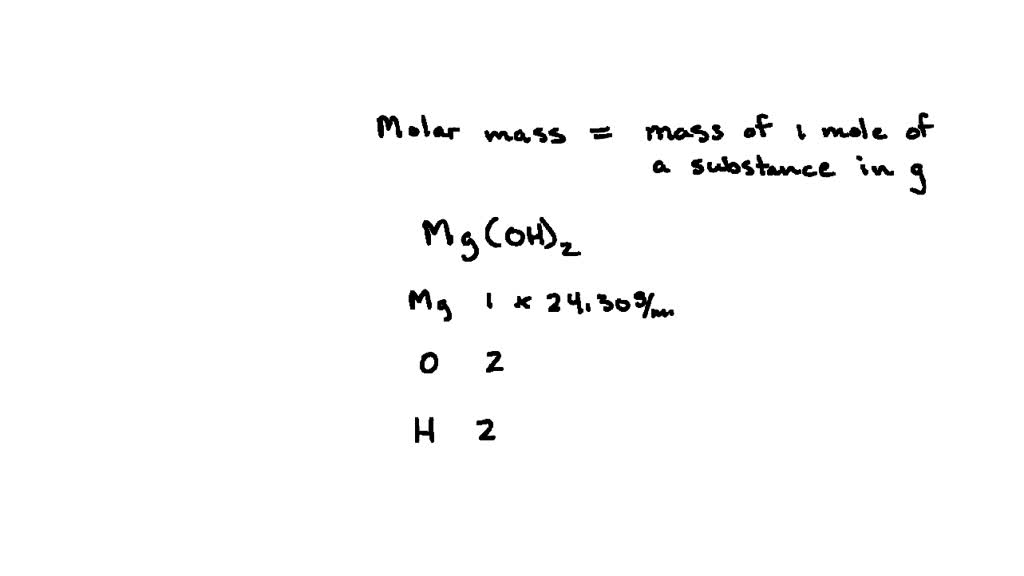SOLVED: The molar solubility of nickel(II) hydroxide (Ni(OH)2) is 2.8 x 10-6 mol/L in pure water at 25°C. What is the molar solubility of nickel(II) hydroxide in 0.050 M NaOH at 25°C? (

Ni(OH)2@Ni core-shell nanochains as low-cost high-rate performance electrode for energy storage applications | Scientific Reports

Realizing Two-Electron Transfer in Ni(OH)2 Nanosheets for Energy Storage | Journal of the American Chemical Society

Calculate the molar solubility of Ni(OH)2 in 0.10M NaOH. The ionic product of Ni(OH)2 is..... - YouTube

Ni(OH)2@Ni core-shell nanochains as low-cost high-rate performance electrode for energy storage applications | Scientific Reports
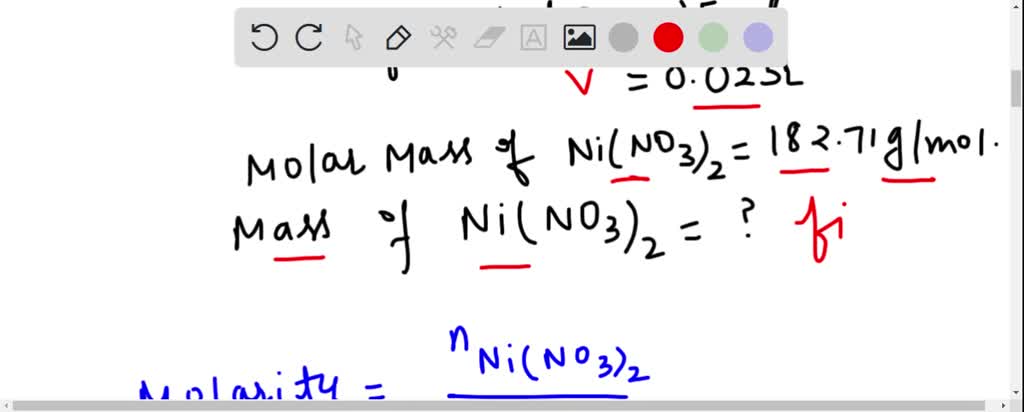
SOLVED: What is the mass of nickel(II) nitrate (182.71 g/mol) Ni(NO3)2 dissolved in 25.0 mL of 0.100 M solution? 4.00810.457 g 45.7 0.250 B 457 g

A mixture of 100mmol of Ca(OH)2 and 2g of sodium sulphate was dissolved in water and the volume was made up to 100mL. The mass of calcium sulphate formed and the concentration
Unanswered Question 5You AnsweredCorrect Answer0/1 pts554. mL of 0.652 M KOH(aq) is mixed with 518. mL - brainly.com

Ni(OH)2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials | Journal of the American Chemical Society
✓ Solved: Approximately 0.14 g of nickel(II) hydroxide, Ni(OH) 2(s) , dissolves per liter of water at...